Nitrogen oxide (NOx) emissions contribute to the formation of acid rain and urban smog. Being highly noxious, they are strictly regulated by environmental agencies. Therefore, cement plants are required to reduce such emissions in their combustion processes through process optimisation and other measures.
Nitrogen oxides (NOx) are generated in almost all combustion processes. Depending on the emissions by its cement plants and the local legislation, the company may have to invest in expensive mitigation solutions or face the payment of fines and a possible production stop.
To be competitive and comply with local regulations, cement manufacturers implement innovative solutions to minimise NOx emissions, either through the treatment of exhaust gases or through the optimisation of the combustion process. Furthermore, it is not only a matter of mitigating NOx emissions but also of maintaining operational efficiency at a viable cost.
Brazil-based combustion specialist Dynamis has been investigating, in close cooperation with cement plants, process optimisation and low-NOx combustion systems to help reduce NOx emissions.
Commercial computational fluid dynamics (CFD) codes as well as in-house developed models and software are used to better understand the process.
NOx formation and destruction
Three main mechanisms govern NOx generation inside a clinker kiln:
- Thermal mechanism – in which N2 from air reacts with oxygen, producingNOx.
- Fuel mechanism – in which the fuel molecule that contains nitrogen breaks into NO, HCN and NH3. The last two react with O2, generating NOx.
- Prompt mechanism – in which fast reactions occur between nitrogen, oxygen and hydrocarbon radicals (CHi), producing NOx.
After the two main NOx formation mechanisms (thermal and fuel) have been closely analysed, the third mechanism (prompt) may also be taken into account. This is because the prompt mechanism is only important in some specific cases, such as the burning of gaseous fuels or lower- temperature combustion processes.
There are two main mechanisms for NOx destruction in process optimisation (excluding selective non-catalytic reduction or SNCR). One pathway to decrease NOx formation involves the addition of NO-reducing agents (mostly HCN, NHi, H2, CO and char). Another common approach, called “reburning”, consists of adding simple hydrocarbon fuels with little or no amount of nitrogen (mostly CH, CH4, natural gas, C2H2, C2H4 and C2H6). Both techniques require low oxygen atmospheres.
The thermal mechanism occurs more intensely above 1100˚C and the NOx destruction reactions have significant rates above 900˚C. Depending on the gas composition, HCN and NHi molecules can begin to react with O2 to form NOx above 1000˚C, reducing the net destruction of NOx, even increasing the emissions in some cases.
In a cement line, low-NOx techniques such as staged combustion and low-NOx burners can be applied to some extent without the need for additional operating costs. However, when the system does not allow sufficient NOx reduction to comply with local regulations, a more expensive solution must be used.
The lowering of NOx emissions requires a deep understanding of NOx formation/ destruction processes, since the fine analysis of the dominant mechanisms will determine which is the best solution strategy. NOx control mechanisms can vary from operational stability (thus reducing the thermal load of the kiln) to changes in the combustion system, or even installation of SNCR systems (using urea or ammonia solutions) with high operating costs.
Combustion staging (regardless of the fuel) not only avoids NOx formation, but can also create an environment that may reduce the NOx present in the kiln exhaust gases. Hydrogen, simple hydrocarbons, intermediary forms of fuel-bound nitrogen (HCN and NH3), and to some extent even carbon monoxide (CO), are NOx- reducing agents, which, depending on the concentration and temperature, are more or less important for NOx emission reduction.
NOx in cement lines
Clinker production presents some interesting challenges in reducing NOx emissions due to the high temperature involved and its direct impact on product quality. The kiln system – the rotary kiln and preheater tower calciner (if installed) – is the most important source of NOx emissions at a cement plant. Because of the success of modern kilns with preheater towers equipped with calciners, this article will focus mainly on this configuration.
The clinkerisation of raw materials requires high temperatures (up to 2200˚C near the flame) inside the kiln and the fuel encounters a stream of hot secondary air. Meanwhile, the combustion that takes place in the calciner is very different from the one inside the kiln: average temperatures are lower, the fuel can burn for a longer period and often there is a mixture of fuel and raw meal particles to avoid localised high-temperature zones.
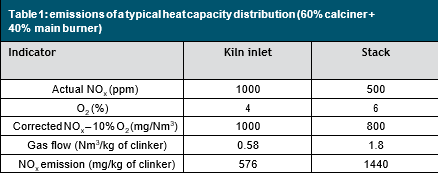
While one might think that the kiln is the only major NOx source in the cement line, a closer look at the usual NOx emission rates from the kiln inlet and stack leads to the conclusion that the calciner is also a significant NOx producer. As Table 1 shows, even though the concentration of NOx is lower on the stack, the specific output of NOx is higher on the stack than on the kiln inlet. In this example, the NOx emission from the kiln is 576mg/kg of clinker and the total emission from the preheater is 1440mg/kg of clinker, resulting in a net production of roughly 860mg/kg of clinker at the calciner. Although these numbers only illustrate one case, the calciner is not to be neglected when addressing NOx emissions.
A closer examination of the kiln burning conditions reveals that there is a restriction on the amount of hot secondary air that enters the flame core, creating a hot under- stoichiometric zone, limiting the formation of NOx at the flame core (due to the lack of oxygen) and promoting an internal reduction of NOx. Though the standard calciner presents a more uniform fuel-air mixture and temperature, it may contain a few hotspots.
On an in-line calciner (ILC) the fuel injection is often localised close to the tertiary air inlet. The fuel stream must meet tertiary air and meal streams in the best possible mixture condition to result in an uniform temperature profile. The use of adequate burners in the calciner can solve the previously-mentioned mixture and uniformisation issue.
On separate-line calciners (SLCs) the fuel is injected in the centre (through a calciner burner) and tertiary air enters tangentially on the combustion chamber. This configuration allows for a hot zone to be created (near the flame), much like the conditions found in the kiln.
One of the most common strategies to reduce NOx is fuel staging. This is effective for two reasons:
- the peak flame temperatures are reduced, which lessens NOx emissions
- the fuel-rich mixture in the primary flame zone also reduces NOx.
Considering only the rotary kiln, when looking at clinker quality, a high peak of temperature is often desirable to allow smaller C3S crystals to be formed. On the other hand, the NOx reduction through process modifications often requires a worsening of the optimal combustion conditions (eg, lower excess air, lower secondary air entrainment, smaller temperature peaks). This indicates that, even using the latest-generation low- NOx burners, there will be some process limitations to the NOx levels that can be reached in the kiln. Inside the calciner there is a greater margin to decrease NOx formation and increase NOx destruction, because in this case the limiting factor is the quality of combustion and not the quality of the product.
In the calciner most of the difficulty in establishing conditions that prevent the formation of NOx relates to achieving the complete combustion of the fuel used.
Models and tools
Dynamis used CFD to model and analyse in detail the combustion reactions inside rotary kilns and calciners. Understanding what happens in the different stages of each system is key to better deciding which technique (and where) should be used.
For CFD simulations, Dynamis uses Fluent and CFX, developed by Ansys – one of the main global computer-aided engineering (CAE) software suppliers.
The NOx modelling requires complex iterations between gas phases and solids (fuel and/or raw meal) to determine how local conditions can influence NOx emissions. Correctly modelling the calcination of raw meal is very important, as it is a highly endothermic reaction that has a considerable impact on the local temperature profile. As seen in different calciner simulations, the lack of homogeneity in the mixture of fuel, air, gas and meal streams makes the thermal mechanism of NOx formation as important as the fuel mechanism.
In addition, calibrating the models (HCN/NH3/NO formation according to the fuel used) has a direct impact on the simulation, and requires extensive knowledge and site measurements. Analytical models and software developed by Dynamis were also used in conjunction with CFD to improve the accuracy of the simulations.
NOx reduction at a cement plant
At the start of 2017 Dynamis was hired by an international cement company to supply a new secondary combustion system, aimed at reducing NOx emissions by 25 per cent. Site measurements indicated a concentration of 782ppm of NOx at the preheater tower outlet at five per cent O2.
The main calciner fuel was pulverised coal, which was originally injected from two f200mm ducts positioned in the front side of the calciner. The calciner also used RDF, fed through two meal inlets.
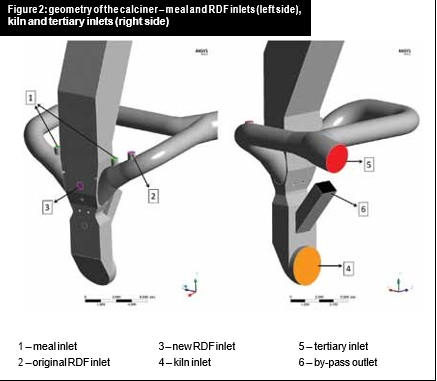
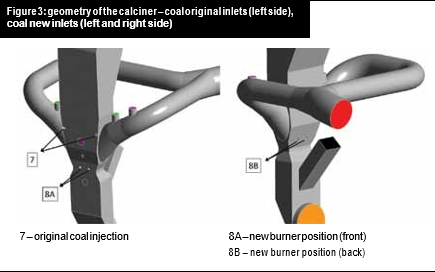
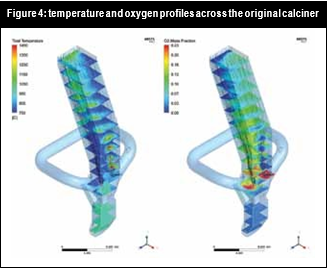
Hot air was entering the system through the tertiary inlet and flue gases from the kiln. All the inlets and outlets are shown in Figure 2 and Figure 3. A CFD analysis of the case showed that with the original geometry, the fuel was injected on a region with high O2 availability and poor reactant distribution, leading to temperature peaks that favoured NOx formation across the calciner.
Dynamis proposed a new geometry for the coal injection through four new burners: two placed at the front side of the calciner and two at the back side, as depicted in Figure 3.
This modification, combined with a repositioning of the RDF injection predicted:
- combustion staging, starting the burning process in a fuel-rich region, generating less fuel NOx and reducing some of the NOx from the kiln gases
- improved mixing of fuel particles and flue gases from the kiln before the tertiary air inlet
- improved uniformity of reactants and temperature (reducing the hotspots).
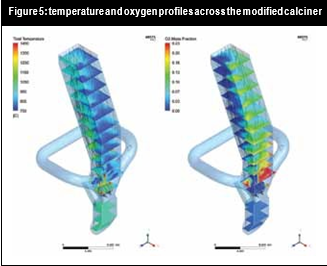
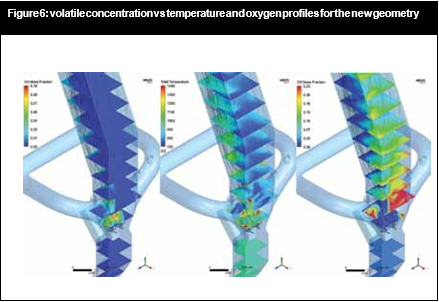
All these changes in flow and chemical reaction behaviour contributed to a reduction of the NOx concentration in the flue gas. Figure 5 and Figure 6 illustrate how it was possible to create a strongly-reducing atmosphere with high volatiles, CO and temperature zone with the new burner configuration near the calciner throat. In this zone the conditions were favourable to reduce the NOx contained in the kiln gases.
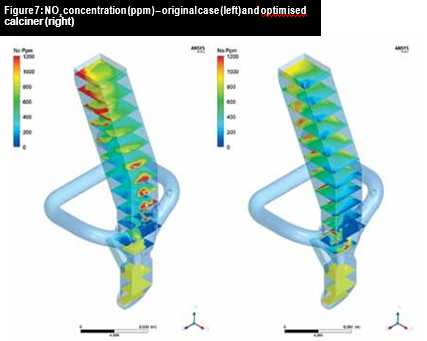
In addition, there was an improved mixing of fuel and flue gases before the tertiary air inlet and hotspots were minimised. Figure 7 contains CFD predictions for NOx concentration (in ppm) inside the calciner for both original and final geometries. A reduction of 25.6 per cent in NOx concentration was calculated at the outlet surface.
Since the coal was to be introduced in a low-O2 environment, Dynamis used its new D-C burners to help promote complete mixing of the fuel and gases, enhance combustion and achieve the expected results.

These modifications were introduced in December 2017 and yielded the expected results. The NOx at the preheater outlet fell from ~1100mg/Nm3 to ~780mg/Nm3 (at 10 per cent O2), as predicted by the CFD, enabling the cement plant to reduce its NOx footprint. The quality of combustion was also enhanced, as CO concentration measurements indicated.

